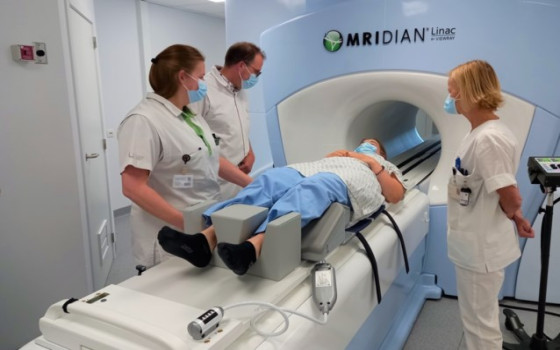
. Its implementation began today... a new framework for health security in the European Union

- Europe and Arabs
- Sunday , 25 December 2022 21:50 PM GMT
Brussels: Europe and the Arabs
As of today, the building blocks of the European Health Union are officially in place. This includes not only stronger EU rules on serious cross-border threats to health, but also a stronger mandate for the European Center for Disease Prevention and Control (ECDC) and a new emergency framework for medical countermeasures.
Combined with the expanded mandate of the European Medicines Agency (EMA) and the creation of the Health Emergency Preparedness and Response Authority (HERA), the EU now has the tools to better prepare for and respond in the event of a public health emergency.
This came in a statement issued by the headquarters of the European Commission in Brussels, in which it also stated,
The entry into force of these new rules complements the European Health Union's preparedness and response capabilities, creating a strong legal framework to improve EU capacity in the critical areas of prevention, preparedness, surveillance, risk assessment, early warning and response.
Serious cross-border threats to EU health regulations now grant:
Strong readiness planning and a more integrated monitoring system
Better ability to accurately assess risk and target response
Solid mechanisms for joint procurement of medical countermeasures
Possibility of adopting joint measures at EU level to address cross-border health threats in the future
Not only can a stronger ECDC issue recommendations to Member States regarding preparedness for health threats, but it can also host a new network of excellence in the EU reference laboratories and set up an EU health task force for rapid health interventions in the event of a major outbreak.
To be effective and operational in times of public health emergency, the Emergency Framework Regulation now allows for the creation of a Health Crisis Management Board within HERA. This Council will rapidly coordinate at EU level the provision of and access to medical countermeasures. The regulation also enables the activation of EU emergency research facilities, emergency research and innovation schemes, and access to emergency funding.









No Comments Found